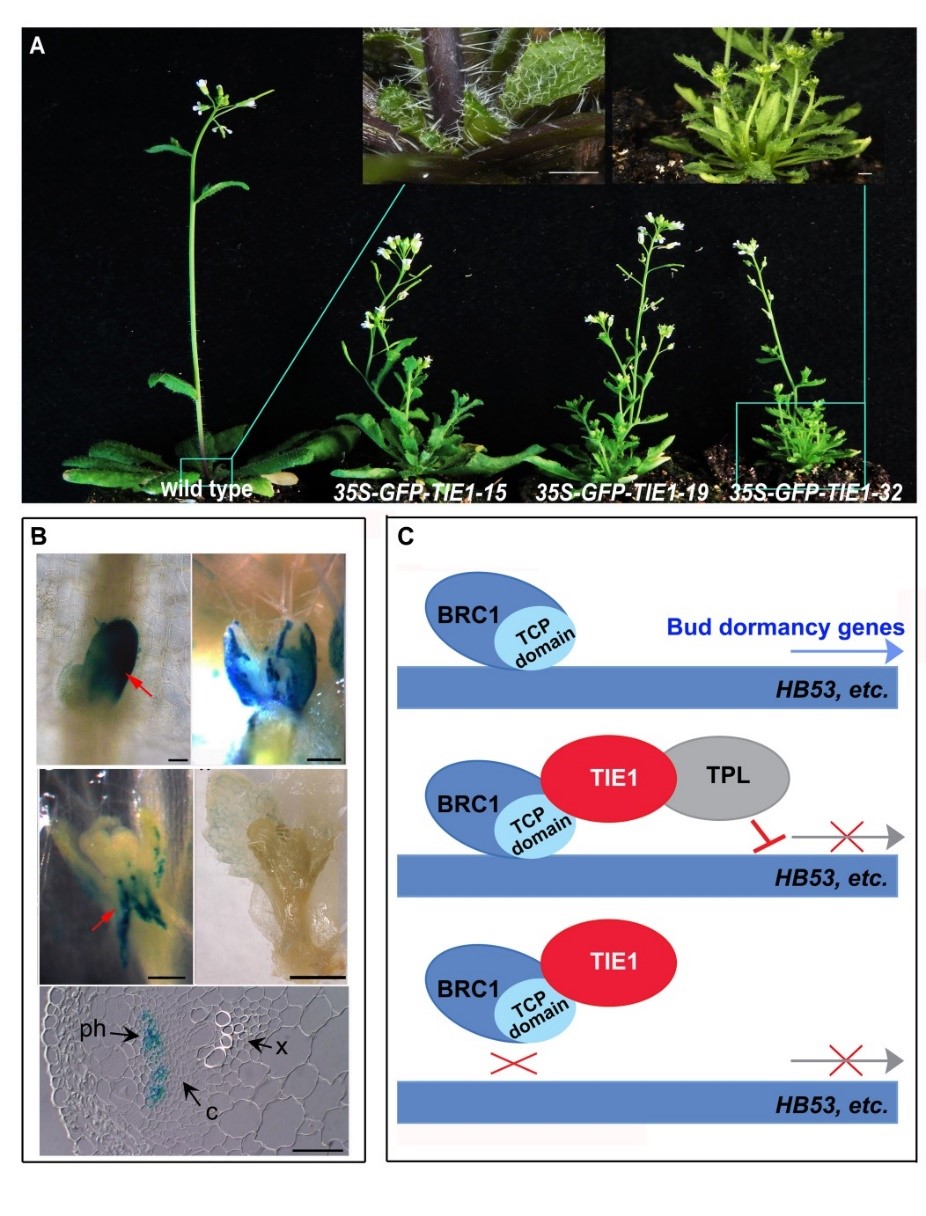
 May 26,2018
May 26,2018
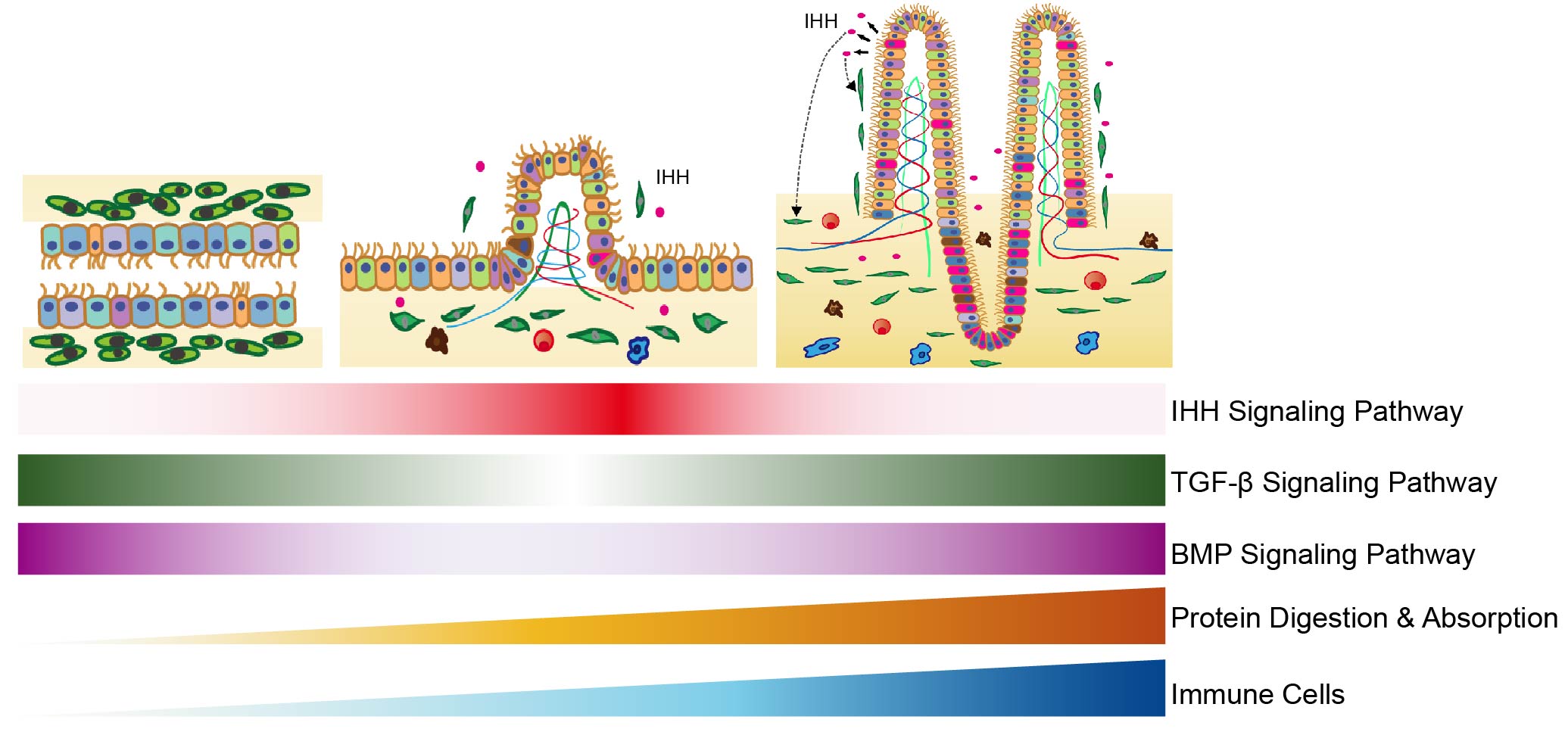
The development of the digestive tract is critical for proper food digestion and nutrient absorption. Here, we analyse the main organs of the digestive tract, including the oesophagus, stomach, small intestine and large intestine, from human embryos between 6 and 25 weeks of gestation as well as the large intestine from adults using single-cell RNA-seq analyses. In total, 5,227 individual cells are analysed and 40 cell types clearly identified. Their crucial biological features, including developmental processes, signalling pathways, cell cycle, nutrient digestion and absorption metabolism, and transcription factor networks, are systematically revealed. Moreover, the differentiation and maturation processes of the large intestine are thoroughly investigated by comparing the corresponding transcriptome profiles between embryonic and adult stages. Our work offers a rich resource for investigating the gene regulation networks of the human fetal digestive tract and adult large intestine at single-cell resolution.