Oct 11,2016
Oct 11,2016
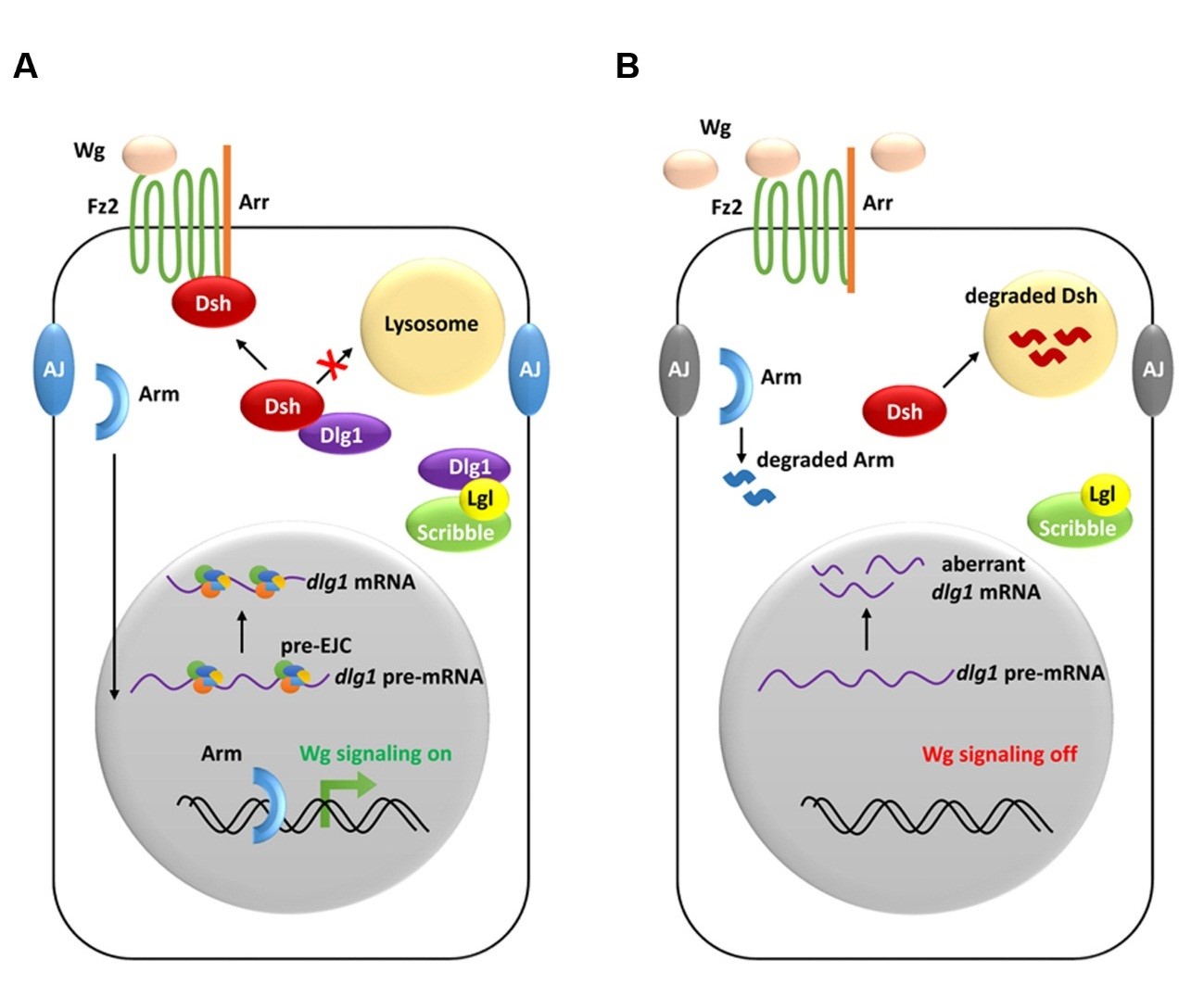
Abscisic acid (ABA) is an essential hormone for plant development and stress responses.ABA signaling is suppressed by clade A PP2C phosphatases, which function as key repressors of this pathway through inhibiting ABA-activated SnRK2s (SNF1-related protein kinases). Upon ABA perception, the PYR/PYL/RCAR ABA receptors bind to PP2Cs with high affinity and biochemically inhibit their activity. While this mechanism has been extensively studied, how PP2Cs are regulated at the protein level is only starting to be explored. Arabidopsis thaliana RING DOMAIN LIGASE5 (RGLG5) belongs to a five-member E3 ubiquitin ligase family whose target proteins remain unknown. We report that RGLG5, together with RGLG1, releases the PP2C blockade of ABA signaling by mediating PP2CA protein degradation. ABA promotes the interaction of PP2CA with both E3 ligases, which mediate ubiquitination of PP2CA and are required for ABA-dependent PP2CA turnover. Downregulation of RGLG1 and RGLG5 stabilizes endogenous PP2CA and diminishes ABA-mediated responses. Moreover, the reduced response to ABA in germination assays is suppressed in the rglg1 amiR (artificial microRNA)-rglg5 pp2ca-1 triple mutant, supporting a functional link among these loci. Overall, our data indicate that RGLG1 and RGLG5 are important modulators of ABA signaling, and they unveil a mechanism for activation of the ABA pathway by controlling PP2C half-life.